Drug Design Approach
Our lead drug candidates, lasofoxifene and ATH-1105, are novel, small molecule therapies with the potential to address devastating diseases where current treatment options are limited. Subject to ongoing clinical testing, we believe our small molecules have the potential to modify the course of disease for patients.
Targeting ESR1-Driven Endocrine Resistance in Metastatic Breast Cancer
Estrogen receptor-positive (ER+) breast cancer is driven by estrogen receptor signaling. Endocrine therapies are initially effective, but resistance frequently develops in metastatic disease.
A major mechanism of resistance is the acquisition of activating mutations in the estrogen receptor gene, ESR1.
Emergence of ESR1 Mutations in ER+ Breast Cancer
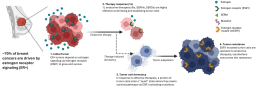
ER+ tumors depend on estrogen receptor signaling for growth and survival. First-line endocrine therapies suppress this signaling by reducing estrogen levels or inhibiting receptor activity. With continued treatment pressure, tumor cells adapt by acquiring ESR1 activating mutations that render them resistant to endocrine therapy, leading to tumor persistence and progression despite therapy.
Lasofoxifene Inhibits Mutant Estrogen Receptor Signaling
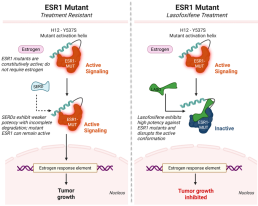
Lasofoxifene is a selective estrogen receptor modulator (SERM) with activity against both wild-type and mutant ESR1. Structural features of lasofoxifene disrupt the active conformation of mutant estrogen receptors.
This inhibition blocks downstream transcriptional signaling and suppresses tumor growth, even in the presence of ESR1 activating mutations.
By directly targeting ESR1-driven resistance, lasofoxifene addresses a critical unmet need in metastatic ER+ breast cancer.
Targeting the Neurotrophic HGF System in Neurodegenerative Diseases
Neurotrophic signaling pathways play a critical role in maintaining neuronal health and function. Hepatocyte growth factor (HGF) has long been recognized as a potential therapeutic target for neurodegenerative diseases, including amyotrophic lateral sclerosis (ALS).
HGF signaling through its receptor, MET, on neurons and glial cells initiates intracellular pathways that counteract neurodegeneration and promote neuronal survival and repair.
Strong evidence from literature supports modulation of the HGF pathway in ALS, demonstrating improvements in motor function, preservation of motor neurons, and delayed disease progression.
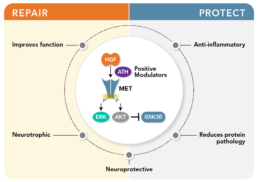
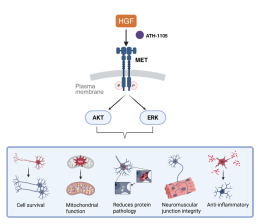
ATH-1105: A CNS-Penetrant Positive Modulator of HGF as a potential treatment for ALS
ATH-1105 is a small-molecule, CNS-penetrant positive modulator of HGF designed to enhance endogenous neurotrophic signaling in the nervous system.
ATH-1105 increases HGF activity and promotes activation of MET signaling, leading to engagement to downstream pathways that support neuronal health and repair.
Enhancement of HGF–MET signaling through ATH-1105 initiates multiple neuroprotective processes relevant to ALS pathology.
Select Scientific Publications on neurotrophic HGF and its Role in Neurodegenerative Diseases
Click below to see links. By clicking on the links below, you will leave LeonaBio’s website. LeonaBio does not endorse or update information on such third-party websites.
- HGF and MET in the Brain and Neurological Disorders Review (Desole et al, 2021)
- HGF General Review (Nakamura and Mizuno et al, 2010)
- HGF Neurotrophic Review (Funakoshi and Nakamura, 2011)
- HGF suppresses disease progression in ALS rat mode
- HGF improves motor function and protects neuromuscular system in ALS mouse model
- HGF delays disease progression and improves neuronal survival in ALS mouse model
- HGF delivery is beneficial in TDP-43 mouse model of ALS
Select LeonaBio Scientific Publications
By clicking on the links below, you will access PDF reprints of peer-reviewed publications made available by scientific journals via open-access.

